The only ISO quality standard specific to the high-risk medical device industry is ISO 13485:2016. Explore the critical shortcomings of ISO 9001 within the healthcare industry and why being “ISO-certified” isn’t enough. Part 2 of a 4-part series exploring the gaps surrounding medical equipment quality.
What You Can’t See Can Hurt You (and Your Patients)
In the first quality article, we talked about a growing problem in many healthcare organizations: How do you ensure all your vendors and service partners are committed to patient safety and quality? In this article, we’re going to focus on the answer. It starts with how you evaluate your vendor’s compliance to quality management system regulations such as those put forth by the International Organization of Standardization (ISO).
What You Might Not Know about ISO
Globally, quality management systems in a variety of industries are based on ISO standards. Some healthcare organizations don’t understand that there are different ISO standards specific to different industries. The only ISO quality standard specific to medical devices is ISO 13485:2016. This standard is based on evaluating the risk to patient safety throughout the life cycle of a medical device.
“We’re ISO-certified” might not provide the quality and patient safety assurance you think it does when it comes to medical device service and rental providers.
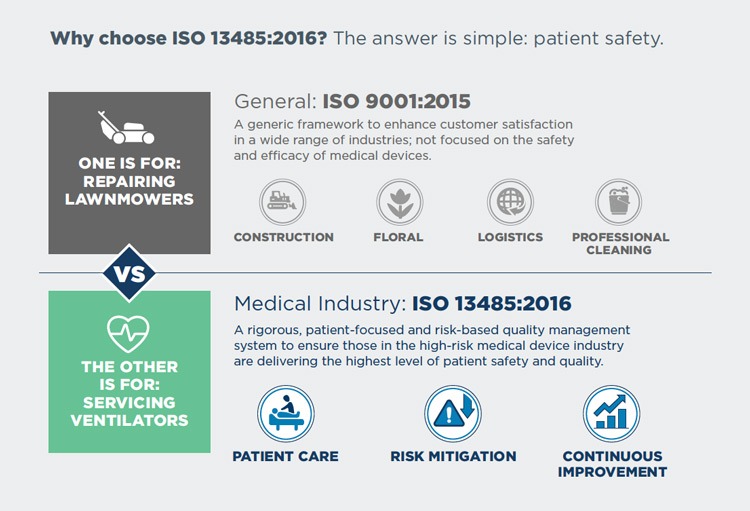
The troubling reality is that while a select few vendors now self-adhere to critical, risk-reducing ISO 13485:2016 standards for their medical equipment management, most are either not certified or are certified to a less stringent ISO 9001:2015. Understanding the difference between ISO 9001:2015 and ISO 13485:2016 is important for you as you select third-party service providers.
So what is ISO 13485?
ISO 13485 is a patient-focused and risk-based quality standard designed specifically for the high-risk medical device industry. The ISO 13485 standard grew out of the more generic ISO 9001 standard — which provides a general framework to ensure quality and enhance customer satisfaction across a wide range of industries.
The Growing Gap Between ISO 9001:2015 and ISO 13485:2016
Healthcare has changed profoundly in the 20+ years since ISO 13485 diverged from the generic ISO 9001. The gap between the two ISO standards has also grown. ISO 13485 is continually updated to account for the evolving risks associated with medical devices. The latest iteration, ISO 13485:2016, is already a mandate for medical device quality assurance. In fact, the FDA is evaluating how to implement ISO 13485:2016 for the regulation of quality management systems associated with medical devices. The updated standard includes additional requirements around rigorous staff training, patient risk-based processes and protocols, service and maintenance. Many of these standards are simply absent in ISO 9001:2015.
It begs the question: Would you let your landscaping company repair your medical devices?
To apply the ISO standard simply, imagine this: Would you trust a landscaping company to service your essential medical equipment? How about a florist or apparel retailer? Of course not. ISO 9001:2015 helps landscapers, florists and retailers to build quality practices and deliver consistent customer service, but it does not pertain to medical devices, patient care or risk mitigation. The point is, you want a vendor who is qualified for the right thing in the right industry. That’s why it’s critical to understand the sometimes-cryptic ISO nomenclature, so you can properly evaluate vendors and protect the performance of your quality engine.
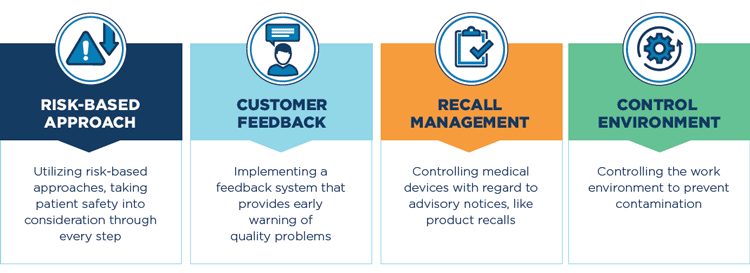
Hold Your Vendors to the Highest Quality Standards
As we explained in the first blog post of this series, small details around quality and patient safety fuel an engine that positively drives every aspect of an organization — from staff satisfaction to patient outcomes and even the bottom line. It’s critical to seek out third-party vendors that demonstrate a commitment to the highest quality and patient safety standards — because every input to the quality engine plays a role in keeping that engine running smoothly.
There are more than 80 key differences between ISO 13485 and ISO 9001. To dive into a few of these differences, check out this simple infographic.
When a vendor tells you, “We’re ISO-certified,” or, “We follow ISO standards,” that doesn’t go far enough. To protect your patients and support the quality outcomes that drive your organization’s success, you need to confirm that vendors who work with medical devices are compliant with ISO 13485:2016 — and seek out vendors that self-adhere to these medical device, risk-reducing standards.
Read the Full Report Today
Want to learn more? You can download the full report today — from the nuances in ISO standards and how they impact patient safety, to tips for how to choose vendors that best support the delivery of quality clinical outcomes and exceptional patient experiences.
Visit our Resource Center to discover more, or return to the blog menu.