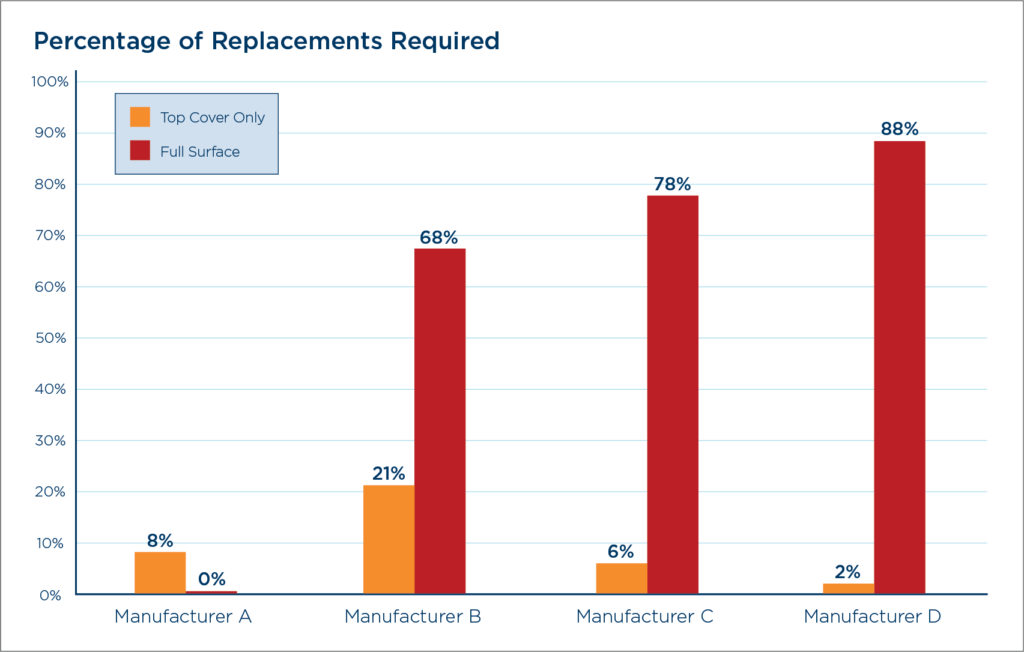
Surfaces requiring full replacement:
85%
Surfaces from other market leading manufacturers (B, C, D)
0%
Surfaces with unique construction from Manufacturer A
Background
Fifty percent of acute care support surfaces are compromised within 3.8 years with an increase in failure odds of 67.6% with each additional year of age.1 The FDA recommends regular inspection of the top cover and internal components for fluid ingress and damage and replacing damaged covers and surfaces to reduce the risk of infection to patients.2,3,4,5
Problem
Risk for cross-contamination from compromised surfaces was shown to be 5.83 times that of controls.5 Replacing surfaces is costly. Preserving their integrity prevents not only infections but also unnecessary spend.
Methods
Surfaces were inspected to observe top cover and internal component damage/staining.3 A top cover with holes/tears or internal staining is recommended for replacement.3 Once the top cover was removed, internal components were inspected for damage/staining/compression and recommended for replacement if observed. Various surfaces were selected to understand how different constructions impacted surface longevity.
Interventions
A unique surface construction was assessed to understand if construction materials/methods decreased fluid ingress/cross-contamination and improved surface lifespan.
Significant Differences Between Typical and Unique Construction:
Typical Construction



Major Acute Care Support Surface Manufacturers top cover immersed in bleach for 5 days resulting in full delamination
Unique Construction



Manufacturer A’s top cover immersed in bleach for 10 days resulting in no delamination
Construction



Major Acute Care Support Surface Manufacturers top cover immersed in bleach for 5 days resulting in full delamination
Unique
Construction



Manufacturer A’s top cover immersed in bleach for 10 days resulting in no delamination
Results
Across 24 facilities, 422 surfaces from various manufacturers with an average age of 6 years were inspected:
- Thirty-seven (37) surfaces (average age 5 years) contained a welded shield* to prevent fluid ingress if the top cover was damaged
- None of these surfaces required full replacement, and
- only 8% (3) required new top covers
- Of the remaining 374 without a welded shield, 85% (318) sustained internal damage requiring full surface replacement
Frequent Damage Seen With Other Leading Manufacturers

Construction: Top cover material is eroded by harsh chemical cleansers
Result: Degraded waterproofing material allows for fluid ingress

Construction: Sewn seams create thousands of holes in cover
Result: Holes allow fluid to permeate the interior components

Construction: Fiberglass fire barrier breaks down over time and is chemically treated
Result: No longer offers fire protection

Construction: Unprotected interior components
Result: Fluid ingress and contamination of interior components, requires full replacement of the surface for patient safety
Damage Prevented With Unique Support Surface Construction

Construction: Specially formulated top cover fabric
Result: No delamination of waterproofing, maintains integrity, and no fluid ingress
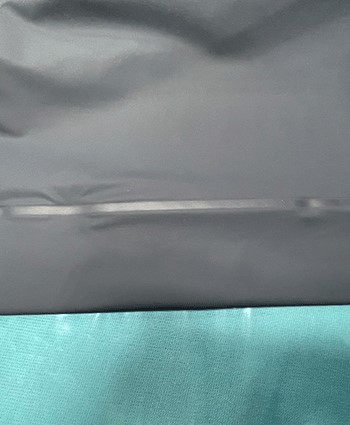
Construction: RF-welded seams permanently bond the material on a molecular level
Result: Avoiding holes to join fabric prevents fluid intrusion
Construction: Fiberglass-free fire barrier
Result: Remains intact over time ensuring adequate fire protection

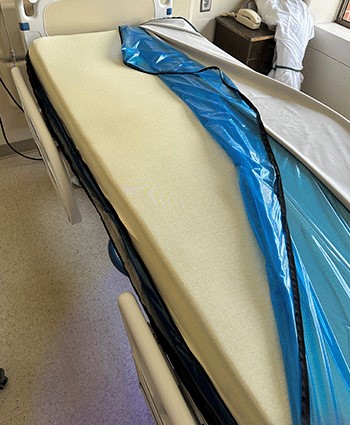
Construction: Welded shield protected interior components
Result: The welded shield prevents fluid ingress to interior components if top cover would get damaged, further preventing full replacement
Implications for Practice
Unique materials and construction prevented fluid ingress and contamination of surfaces preserving longevity of costly assets:
- Previous studies have shown that most surfaces sustain internal damage triggering replacement in less than five years
- None of the surfaces in this study with a welded shield required full replacement—only three top covers (~$1,300 less than cost of a new surface)
Taking this analysis further, the cost of replacing 318 surfaces would be ~$477,000—where replacing 8% of top covers only would be ~$6,000.
- Surfaces with a welded-shield construction yield significant cost savings
- Considering at 5 years the surfaces with welded shields did not require replacement, inspection of these surfaces beyond 5 years is warranted to understand total useful life
Additional Spend Required for Patient Safety
In a 400 Bed Hospital by Year 5
| Manufacturer | Surfaces Requiring Replacement | Cost |
| A | 0% | $0 |
| B | 68% | $408,000 |
| C | 78% | $468,000 |
| D | 88% | $528,000 |
A= Agiliti®
B-D = Other market leading surface manufacturers
*Core Shield™ by Agiliti

